The chemical formula SO2 represents the chemical compound Sulfur Dioxide. The substance is a colorless gas with a recognizable pungent odor similar to the smell of a burnt matchstick.
A large quantity of SO2 is released during volcanic eruptions. It is also found in some hot water springs. Sulfur Dioxide contributes to global warming as a proponent of the greenhouse effect. This is evidenced by the sulfur cycle observed on Venus, where it forms clouds of Sulfuric acid. Trace amounts of the compound have also been observed on other bodies in the solar system.
Sulfur Dioxide is manufactured on an industrial scale by burning or roasting Sulfur and its components (Sulfide ores, Sulfites) in the presence of Oxygen. The gas released is captured and primarily used in the production of Sulfuric Acid through the contact process. Here, SO2 is converted to Sulfur Trioxide, which combines with sulfuric acid to give Oleum (disulfuric acid). A combination of Oleum with water gives Sulfuric Acid. Billions of kilograms of SO2 are produced annually to meet global requirements.
In addition to its wide industrial use, SO2 is also used as a preservative in dried fruits, a reagent in the laboratory, and various biomedical applications. It is also being looked at as a potential refrigerant and as a tool for climate engineering.
As an irritant, it must be handled with care. Prolonged exposure can lead to respiratory illnesses.
SO2 has the following properties:
| Name of the compound | Sulfur Dioxide (SO2) |
| No. of valence electrons | 6 + (6 x 2) = 18 valence electrons |
| Hybridization of the central atom | sp2 |
| Bond Angles | 119° |
| Molecular Geometry of SO2 | Bent Molecular Geometry |
Contents
SO2 Valence Electrons
To form the Lewis structure of Sulfur Dioxide, we need first to determine the number of valence electrons available. These valence electrons act as the building blocks of the structure. They are found in the atom’s outermost shell, where the force of attraction from the nucleus is the weakest. As such, they can potentially break free to take part in bond formation and exchanges.
Each constituent atom in the compound contributes a set amount of valence electrons to the overall structure. There are two oxygen atoms and one sulfur atom present in SO2.
Sulfur is in group 6(Chalcogens) of the periodic table with the electronic configuration [Ne] 3s²3p⁴. Therefore, the Sulfur atom contributes 6 x 1 = 6 valence electrons
Oxygen has six valence electrons (group 6) and has a valency of -2. Oxygen’s electronic configuration is 1s22s22p4. Therefore, the two Oxygen atoms present contribute 6 x 2 = 12 valence electrons.
Thus, the total number of valence electrons available to form [SO2] is given by:
6[S] + 12[O] = 18 valence electrons.
SO2 Lewis Structure
Now that we’ve calculated the number of valence electrons available to us, we move on towards building up the Lewis structure for SO2.
Sulfur is the least electronegative atom in the compound and will act as the central atom. The two oxygen atoms are arranged adjacent to the central sulfur atom. From the 18 valence electrons available, four are used to form covalent bonds between Sulfur and Oxygen atoms. This arrangement is shown in the figure below.


The remaining valence electrons are then used to fill the outermost shells of the Oxygen atoms on the outside. This arrangement leaves us with a lone pair on the central sulfur atom, as shown below.
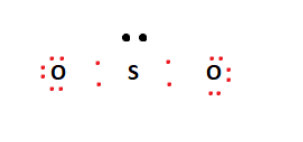
The two oxygen atoms’ octet requirements have been met; however, this is not the case for Sulfur. We form a double bond between one of the oxygen atoms and the central sulfur atom to address this. This double bond meets the sulfur atom’s octet requirements, seemingly giving us our SO2 Lewis structure.

To verify the stability of this Lewis structure, we can calculate its formal charges.
Formal charges for an element/structure help determine its most stable Lewis Structure state. It is determined such that the elemental charge on each atom is closest to zero.
FC = Valence Electrons – Non-bonding electrons – (Bonding electrons ÷ 2)
In this case,
| Element | V | N | B/2 | FC |
| S | 6 | 2 | 6/2 | +1 |
| O | 6 | 6 | 2/2 | -1 |
| O | 6 | 4 | 4/2 | 0 |
The formal charges from the above table are represented graphically below. Either of the Oxygen atoms can take part in the double bond formation with the central Sulfur atom. This gives rise to resonance structures.
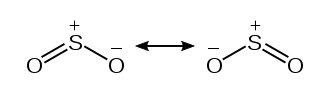
However, in theory, we’d need the formal charges to be as close to zero as possible. By taking advantage of the Sulfur atom’s expanded octet, we can accommodate two double bonds. This arrangement is shown in the figure below.
Let us verify its stability by calculating the formal charges of the constituent atoms present in this arrangement.
In this case,
| Element | V | N | B/2 | FC |
| S | 6 | 2 | 8/2 | 0 |
| O | 6 | 4 | 4/2 | 0 |
| O | 6 | 4 | 4/2 | 0 |
The formal charges being zero in the above table indicate that the double-bonded SO2 arrangement is completely stable. This is a theoretical structure obtained using formal charges- this is the structure that we will take to be Sulfur Dioxide’s final Lewis structure.
However, it is worth noting that in an experimental sense (data and tools), we find single and double bonds present in the SO2 structure.
The final Lewis structure for SO2 is shown below. It can be observed that all of the octet requirements have been fulfilled. The central Sulfur atom has a lone pair attached to it. This is possible due to the Sulfur atom’s expanded octet capability.
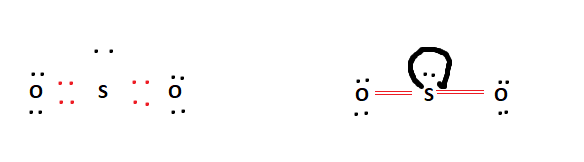
SO2 Hybridization
Sulfur Dioxide comprises a central Sulfur atom surrounded by Oxygen atoms on either side. An easy way to determine the hybridization of a compound is to determine the number of electron domains it possesses.
There are two covalent bonds and one lone pair. There are three electron domains, and this gives SO2 an sp2 hybridization.
Therefore, the hybridization of Sulfur Dioxide is sp2.
SO2 Bond angles
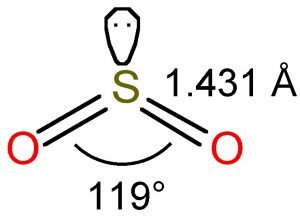
According to the VSEPR theory, the Oxygen atoms are repelled by each other and the lone pair, thus forming a bent molecular shape. As such, the bond angle of SO2 is 119°.
SO2 Molecular Geometry and Shape
To determine the molecular geometry of Sulfur Dioxide, we must observe its Lewis structure. There are two Oxygen atoms bonded to the central Sulfur atom. There is also a lone pair attached to the Sulfur atom. This indicated a bent molecular shape.
We can use the A-X-N concept and its table to verify and determine the molecular geometry of SO2.
‘A’ represents the central atom. The value of ‘A’ here is 1.
‘X’ represents the number of atoms bonded to the central atom. In this case, there are two oxygen atoms bonded to the Sulfur atom.
Therefore, X =2.
‘N’ represents the number of lone pairs attached to the central atom. In this case, N = 1 as there’s a lone pair of electrons attached to the Sulfur atom.
Therefore, that would give us AX2N for the SO2 molecule. From the A-X-N table below, we can determine the molecular geometry.
| Formula | Shape | Bond Angle (Theoretical) |
| AX2 | Linear | 180 |
| AX3 | Trigonal Planar | 120 |
| AX4 | Tetrahedral | 109.5 |
| AX5 | Trigonal Bipyrimidal | 120, 90 |
| AX6 | Octahedral | 90 |
| AX2N | Bent | 120 |
| AX2N2 | Bent | 109.5 |
The AX2N formula corresponds to a bent molecular geometry.
Therefore, Sulfur Dioxide has a bent molecular geometry.
CONCLUDING REMARKS
Let’s quickly summarize the salient features of the Sulfur Dioxide compound:
- SO2 comprises a Sulfur atom surrounded by two oxygen atoms.
- In its most stable state, the Sulfur atom forms double bonds with the adjacent oxygen atoms. There is also a lone pair above the Sulfur atom.
- The hybridization of the SO2 is given by sp2.
- SO2 has a Bent molecular structure and shape with bond angles of 120°.

Nice post. Can you give more explanation about electronegativity and its effects? Also if you give a comparison between SO2 and CO2, then it will help a lot.
Thanks.
sooooooooooo helpful can finally complete my assignment
Why do some images on this page show that both oxygen molecules have a double bond with sulfur. If this is the case, sulfur will have 10 electrons in its valance shell. And moreover, there is no mention of existence of dative bond here.
Some explanation would help a lot,
Thanks
NICE
Outermost shell has 8 electrons. How it is possible for sulphur with 10 electrons
Sulphur can have an expanded octect because the orbitals d.
In that case sulphur shows an expanded octet.
Sulphur can exeed its octet to become stable by acheiving its covalency i.e.,4